CIP Cleaning System
Cleaning In Place : free from disassembling and manually cleaning.

The CIP cleaning system cleans the interior of an appliance by means of pre-installed equipment. A pump delivers cleaning liquid into the appliance, then automatically cleans the interior. Previously appliances of the production equipment were disassembled before being manually cleaned. Compared to this, KIT method makes it much easier. Moreover it is possible to clean multiple lines by only switching the control panel. Consequently its efficiency and safety improve dramatically. KIT offers the best qualification of CIP cleaning system to customers.
Customer’s concept
□We request to clean large equipment at production facilities.
□We request to clean appliances within a containment area without disassembly.
KIT design
□KIT offers CIP cleaning solution by means of in-house nozzles and abundant performance records.
Introduction image

Advantages and effectiveness of introducing CIP
●Available to maintain steady cleaning effectiveness and consequently to improve safe-quality and betterment of the product.
●Through automation, cleaning and manufacturing operation is much improved.
●By omitting disassembly/assembly process of the device, working time is economized and production is improved.
●Compared to manual labor, safety of operations and labor reduction are expected.
●Since there is no individual differences of result and no apprehensions over contamination at the assembly time, improvement of sanitation standard and stability can be realized.
●It is possible to save the consumption of cleaning water, steam and detergent.
●Larger production facilities can be achieved.
●Since cleaning data can be collected, it is possible to verify the cleaning process control and also to inspect wether the cleaning method meets the international standards.
VARIETIES of CIP CLEANING SYSTEM in SOLID PHARMACEUTICAL PREPARATIONS
When cleaning pharmaceutical production lines, pass-through-method(one way method)is mainstream to avoid contamination. However recycling method is still practicable if the risk level is within limits.
CIP SYSTEM
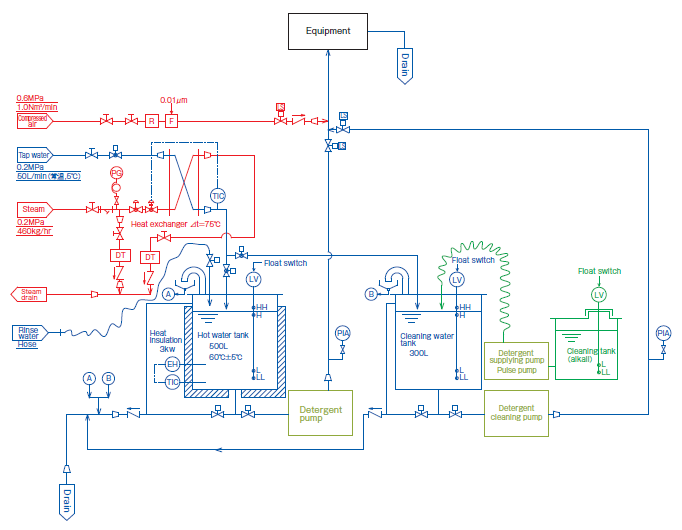
This is a system to clean single pieces of equipment. It is suitable for cleaning large equipment or equipment within the contamination area where manual cleaning is difficult.
It can set up the detergent concentration level or cleaning method depending on the stain type. Furthermore future oriented tackling or function addition is available by means of in-house designing. By installing CIP system closely to the cleaning object, cleaning time and energy consumption are saved.
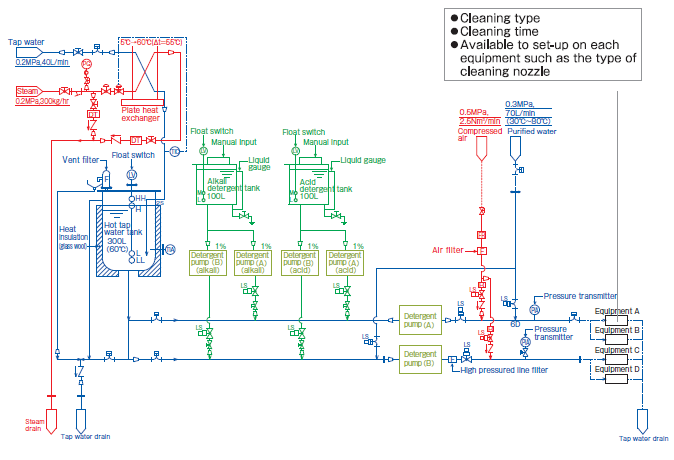
Design image
STATION CIP DEVICE
This is the system to clean multiple equipment guided by one CIP. Since cleaning data of multiple equipment are integrated, centralized control of the lines is possible.
Also reduction of the running cost is expected. It is available to set up the different cleaning menus in accordance with the equipment. It is also available to select the most suitable cleaning method for cleaning type or cleaning time.
Design image